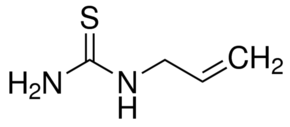
Ceric Sulfate
Cerium(IV) sulfate, also called ceric sulfate, is an inorganic compound. It exists as the anhydrous salt Ce(SO4)2 as well as a few hydrated forms: Ce(SO4)2(H2O)x, with x equal to 4, 8, or 12.
Uses:
The ceric ion is a strong oxidizer, especially under acidic conditions. If ceric sulfate is added to dilute hydrochloric acid, then elemental chlorine is formed, albeit slowly. With stronger reducing agents it reacts much faster. For example, with sulfite in acidic environments it reacts quickly and completely.
When ceric compounds are reduced, so-called cerous compounds are formed. The reaction taking place is:
- Ce4+ + e− → Ce3+
The cerous ion is colorless.
Description
| Properties | |
|---|---|
|
Chemical formula
|
Ce(SO4)2 |
| Molar mass | 332.24 g/mol (anhydrous) 404.304 (tetrahydrate) |
| Appearance | Yellow solid (anhydrous) yellow-orange crystals (tetrahydrate) |
| Density | 3.91 g/cm3 (tetrahydrate) |
| Melting point | 350 °C (662 °F; 623 K) (decomposes) |
| Boiling point | NA |
|
Solubility in water
|
Soluble in small amounts, hydrolyzes in large amounts of water 21.4 g/100 mL (0 °C) 9.84 g/100 mL (20 °C) 3.87 g/100 mL (60 °C) |
| Solubility | soluble in dilute sulfuric acid |
|
Magnetic susceptibility (χ)
|
+37.0·10−6 cm3/mol |
| Structure | |
|
Crystal structure
|
orthorhombic |
| Hazards | |
| Main hazards | Oxidizer |